
Suzhou, China, September 5th, 2025 - Accro Bioscience (Accropeutics), a clinical-stage biotech company focused on molecular mechanisms of regulated cell death and related pathogenesis in human diseases, today announced that it will present results from a Phase II clinical study evaluating AC-201, an oral, selective TYK2/JAK1 Inhibitor, in moderate-to-severe plaque psoriasis, at the European Academy of Dermatology & Venereology (EADV) Congress, being held September 17-20th, in Paris.

Shanghai, China, August 29, 2025 - Accro Bioscience and Fosun Pharma (600196.SH; 02196.HK) today announced that Accro will grant exclusive rights to develop, manufacture, and commercialize its independently developed, highly selective TYK2/JAK1 inhibitor to Fosun Pharma in Greater China (including Chinese Mainland, Hong Kong SAR, and Macau SAR).
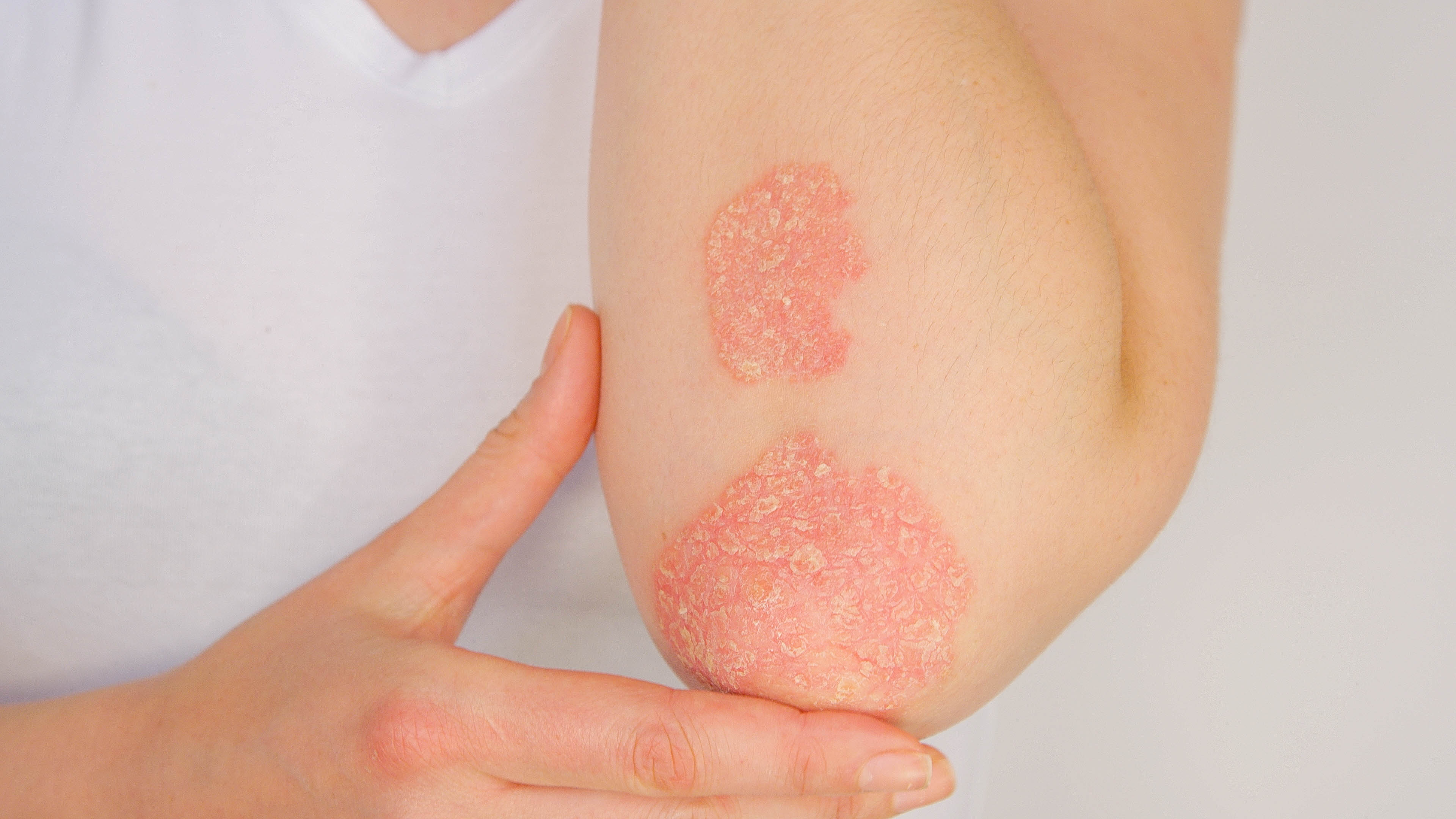
New York and Suzhou, China, May 21st, 2025 -- Accro Bioscience (Suzhou) Limited (Accropeutics), a clinical-stage biotech company focused on molecular mechanisms of regulated cell death and related pathogenesis in human diseases, today announced positive results from their Phase 2 clinical trial of AC-201, an oral, selective TYK2/JAK1 Inhibitor, in moderate-to-severe plaque psoriasis.

New York and Suzhou, China, March 14th, 2025 -- Accropeutics Inc. (Accropeutics), a clinical-stage biotechnology company pioneering the development of novel therapeutics that target molecular mechanisms of regulated cell death for immune mediated diseases, today announced the closing of its $12 million Series B Plus financing.

New York, February 13th, 2025 – Accropeutics Inc. (Accropeutics), a clinical-stage biotechnology company pioneering the development of novel therapeutics that target molecular mechanisms of regulated cell death for immune mediated diseases, today announced the dosing of their first patient in the Phase Ib clinical trial of AC-101. AC-101 is a novel RIPK2 inhibitor being developed for the treatment of moderate-to-severe Ulcerative Colitis (UC).

Company to conduct a Phase II multi-regional clinical trial (MRCT) for the treatment of Ulcerative Colitis (UC)